 News
News
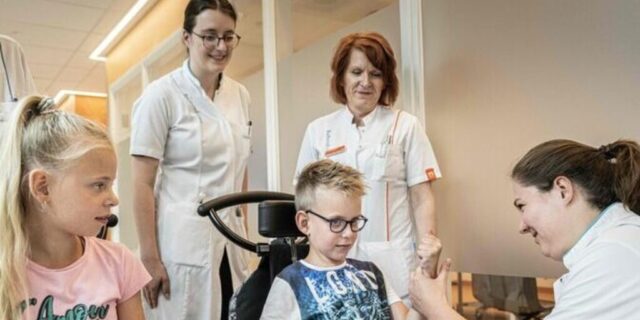
LAMA2-RD support brochure launched for families facing a diagnosis
The Voor Sara Foundation has released a specially designed brochure to support families who have just received a LAMA2-RD diagnosis…
The Voor Sara Foundation has released a specially designed brochure to support families who have just received a LAMA2-RD diagnosis…
As the internationale conference on Lama2 in Instanbul, Türkiye is approaching and we are very much looking forward to seeing…
During the International LAMA2 Conference in Istanbul, a live stream with live translation of the presentations will be available on…
After successful conferences in Maastricht (2019) and Barcelona (2023), we are pleased to announce the third LAMA2-RD-focused conference, to be held…
The Prinses Beatrix Spierfonds, Stichting Voor Sara, CureCMD and Giving Strength are continuing their collaboration with Radboudumc by extending and…
Modalis Therapeutics, the biotechnology company developing novel gene-based therapies for the rare inherited muscle disease LAMA2-MD, has recently published an…
 News
News
 News
News
 News
News
 News
News
 Research updates
Research updates