One step closer: FDA grants Rare Pediatric Disease designation to Modalis Therapeutics’ MDL-101 gene therapy
Modalis Therapeutics recently announced that the U.S. Food and Drug Administration (FDA) has granted Rare Pediatric Disease (RPD) designation to MDL-101, a novel gene therapy for the treatment of LAMA2-CMD.
What is RPD and Why Does it Matter?
The FDA awards Rare Pediatric Disease (RPD) status to therapies being developed for the treatment of life-threatening conditions affecting children under 18 and fewer than 200,000 people in the U.S. This designation offers incentives to developers like Modalis, including potential faster FDA review of other treatments if the drug is approved, accelerating the development of critical therapies for rare diseases.
This milestone is the first of its kind for a gene therapy targeting LAMA2-CMD and therefore marks an important and hopeful step for all those living with LAMA2-CMD and their families. Haru Morita, CEO of Modalis Therapeutics, remarked, “this designation shows our efforts are on the right track. We hope to further accelerate the development of this cutting-edge therapy.”
What’s Next?
Although the RPD designation is a significant milestone, it doesn’t mean the treatment will be available immediately. Drug development is complex and can take time. Dr. Gustavo Dziewczapolski, Scientific Director of CureCMD, emphasized, “there’s no clear timeline for completing pre-clinical studies, setting up clinical trials, and getting regulatory approvals to move the program forward.”
Meanwhile, Modalis Therapeutics have also submitted another application for Orphan Drug designation for MDL-101. The Orphan Drug designation is another FDA special status which can offer additional incentives to accelerate the development of MDL-101, such as tax credits, grant funding and assistance with the regulatory approval process. This application is currently still under review.
Who are Modalis Therapeutics?
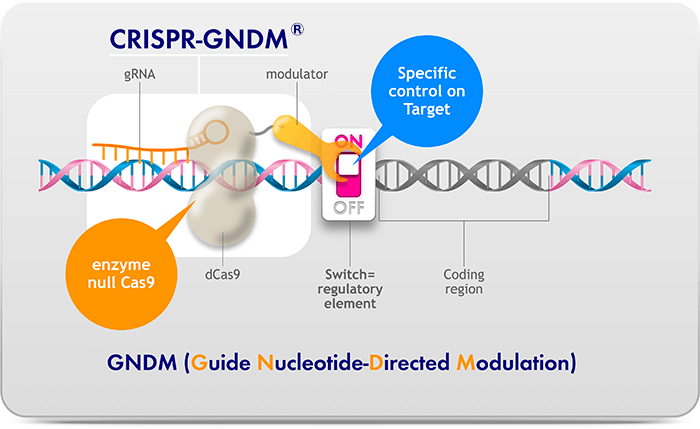
Modalis Therapeutics, based in Tokyo with research facilities in Waltham, Massachusetts, specializes in genetic medicine. Using CRISPR-GNDM® technology, the company develops precision therapies for rare genetic disorders, aiming to improve patient outcomes. For more information, visit their website.
How Does MDL-101 Work?
MDL-101 is a gene therapy which uses CRISPR technology to boost the expression of the LAMA1 gene in muscle tissues, compensating for loss-of-function caused by mutations of LAMA2, and therefore has the potential to provide a one-time, durable treatment to benefit people living with LAMA2-CMD. More information can be found here.
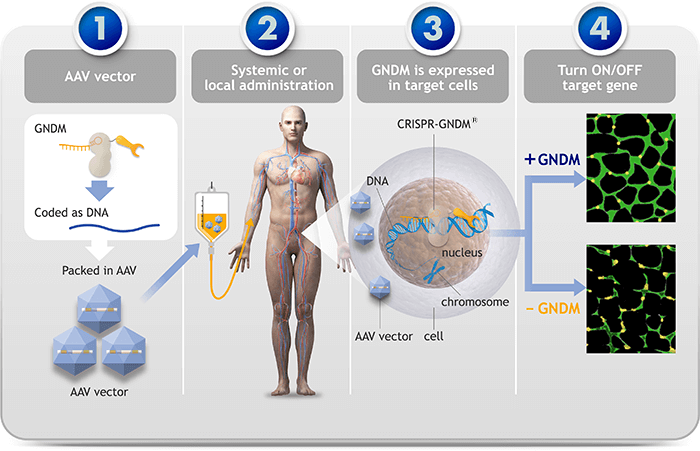
CRISPR-GNDM® technology enables to control gene expressions through modulating a switch on or off.
1) The CRISPR-GNDM® technology is packaged into an AAV vector. 2) The AAV vector is administrated into target tissue locally or systemically. 3) The AAV vector modifies target cells, introducing GNDM DNA which is translated into GNDM protein. 4) GNDM proteins binds to a target gene switch to turn on the LAMA1 gene, to compensate for mutations in LAMA2.